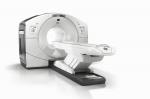
GE Healthcare today announced U.S. FDA 510(k) clearance of its Discovery* IQ PET/CT system, enabling both outstanding image quality and intelligent quantitation to help physicians deliver the best possible patient outcomes.
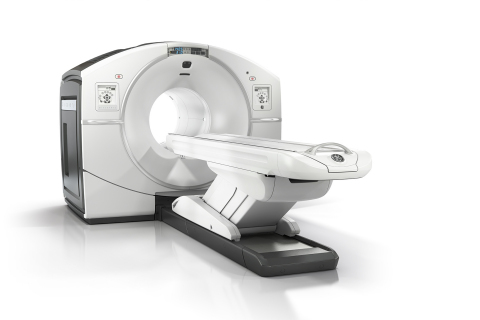
GE Healthcare Discovery IQ (Photo: Business Wire)
Physicians not only want the ability to detect smaller lesions, but also the ability to determine whether the patient is responding to current treatment. Discovery IQ delivers the highest NEMA (National Electrical Manufacturers Association) sensitivity (up to 22 cps/kBq) and the largest axial field-of-view (up to 26 cm) compared to other market leading** PET/CT equipment. This system can image with both half the PET dose and half the scan time.1 Discovery IQ with Q.Clear is designed to provide more accurate quantitation (SUVmean) with excellent image quality (SNR) for small lesion detection, fast and efficient reading, and a confident diagnosis.
“By 2020, it’s estimated that 50% of people will develop cancer at some point in their lives and we also know that currently, approximately 70% of cancer patients do not respond to their initial chemotherapy treatment,” said Wei Shen, general manager of GE Healthcare PET/CT. “I’m excited about the recent FDA clearance of Discovery IQ, which will help physicians achieve their primary mission of delivering the best possible patient outcomes. And, by making Discovery IQ mobile-ready, we engineered it to be accessible to more patients in more places, allowing for high-performance PET/CT clinical care to whoever needs it.”
GE Healthcare’s new Q.Clear technology is a critical component of Discovery IQ. It delivers, for the first time, no trade-off between image quality and quantitative SUV measurements. By providing two times improvement in both quantitative accuracy (SUVmean) and image quality (SNR) in PET/CT imaging, this innovative new tool provides benefits to physicians across the cancer care continuum from diagnosis and staging to treatment planning and assessment.
For more information on Discovery IQ, click here.
* Trademark of General Electric Company
** Market leading PET/CT equipment is defined based on IMV’s Medical Information Division’s IMV 2012 report as the manufacturers representing more than 90% of the US Installed Base.
1 Comparing Discovery IQ 5 ring to a Discovery IQ 3 ring.
About GE Healthcare
GE Healthcare provides transformational medical technologies and services to meet the demand for increased access, enhanced quality and more affordable healthcare around the world. GE (NYSE: GE) works on things that matter - great people and technologies taking on tough challenges. From medical imaging, software & IT, patient monitoring and diagnostics to drug discovery, biopharmaceutical manufacturing technologies and performance improvement solutions, GE Healthcare helps medical professionals deliver great healthcare to their patients.
For our latest news, please visit http://newsroom.gehealthcare.com
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20140922006172/en/
Contacts:
GE Healthcare
Amanda Gintoft,
+1-414-412-7062
Amanda.gintoft@ge.com